1:30min
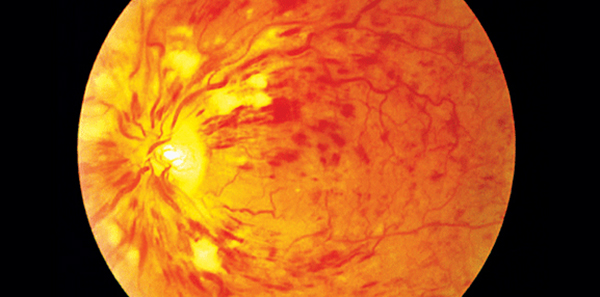
Bayer Australia has announced that Eylea (aflibercept) has been approved by the Therapeutic Good Administration (TGA) for the treatment of visual impairment due to macular oedema secondary to central retinal vein occlusion (CRVO) in adults.
The approval, announced on 4 December 2013, will provide a new treatment option for CRVO and may allow injection intervals to be extended for greater periods of time following the first three monthly injections.
Eylea was first listed on the Pharmaceutical Benefits Scheme (PBS) in December 2012 for the treatment of age-related macular degeneration.
The TGA’s recent approval for use in treating CRVO follows FDA approval in the United States in late 2012 and the backing of the European Medicines Agency in July 2013.
Central retinal vein occlusion affects nearly 30,000 Australians aged 50 years or older.