1:30min
By Rhiannon Riches
Communications Manager
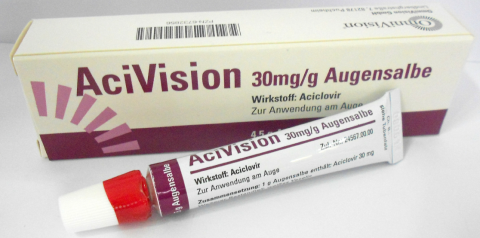
AciVision (Aciclovir) 3% (30mg/g) eye ointment 4.5g has received Therapeutic Goods Administration (TGA) approval for topical application for treatment of ocular herpes.
Optometry Australia understands that an ongoing supply will be available in Australia in the coming weeks.
This follows an announcement that Zovirax (Aciclovir) 3% eye ointment 4.5g, will cease production.
GlaxoSmithKline Australia, the manufacturer of Zovirax (Aciclovir), advised Optometry Australia that stocks in Australia are estimated to last until approximately December 2018.
AciVision is not currently listed on the PBS.
Tagged as: Therapeutics