1:30min
By Rhiannon Riches
Assistant Editor
Eylea
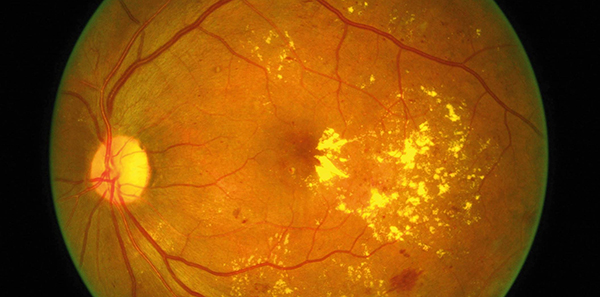
Australians diagnosed with diabetic macular oedema (DME) now have access to a new subsidised treatment option, Eylea (aflibercept).
Eylea, manufactured by Bayer, was made available on the Pharmaceutical Benefits Scheme (PBS) on 1 October.
Professor Paul Mitchell, Head of Ophthalmology at Sydney’s Westmead Hospital, said the PBS listing of Eylea was an important additional therapy option for ophthalmologists and their patients.
‘This means we have another tool available to address this serious and in many cases, life-changing complication associated with diabetes.
‘GPs, diabetes educators and optometrists play an invaluable role in supporting people living with diabetes to have their regular, biannual eye checks and if necessary receive timely treatment to save their sight,’ Professor Mitchell said.
Since 1 October, Eylea has also been available on the PBS for the treatment of visual impairment due to macular oedema secondary to central retinal vein occlusion.
It was already PBS-listed for the treatment of wet age-related macular degeneration.
For more information, visit www.pbs.gov.au.
AciVision
AciVision aciclovir ophthalmic ointment has been listed on the Pharmaceutical Benefits Scheme.
AciVision aciclovir 3% eye ointment, 4.5 g is listed on the PBS, priced at $37.70 for consumers.
Zovirax aciclovir, manufactured by GlaxoSmithKline Australia, is also listed on the PBS in the same quantity for the same price.
Zovirax was recalled in October 2014 due to an issue affecting aciclovir, the product’s active pharmaceutical ingredient, which did not meet Therapeutic Goods Administration specifications and resulted in a retail level recall.
AciVision aciclovir, a German registered product, was temporarily imported to fill the supply shortage following the Zovirax recall, but AciVision was not listed on the PBS and was priced at $80 for consumers.
Optometry Australia had made a submission to the PBS requesting that AciVision be listed.