1:30min
Dr Emily Glover OD
Clinical Resident Optometrist, The Australian College of Optometry, Australia
The University of Melbourne Optometry and Vision Sciences
Dr J James Thimons OD FAAO ABO
Chairman National Glaucoma Society, USA
Medical Director, Ophthalmic Consultants of Connecticut, USA
The ProKera bandage contact lens1 is a therapeutic device used to promote healing of a damaged corneal or conjunctival surface.2 It is used widely in The United States of America, with FDA approval,3 to treat diseases such as severe dry eye, chemical injury, keratitis, herpetic ulcers and recurrent corneal erosions.2

Figure 1A-1F. Biological contact lens insertion
The convenience and efficiency of the biological contact lens makes it desirable to use in clinical practise and has been shown to work successfully in managing recurrent corneal erosions.
The device works by inserting a small portion of amniotic membrane* onto the posterior surface of a ProKera conformer between two polycarbonate rings. This is then applied to the patient’s eye, where the amniotic membrane interacts with the anterior surface to promote healing and regeneration. The amniotic membrane eventually dissolves, usually in about one week, and the ProKera device can be removed by the optometrist.
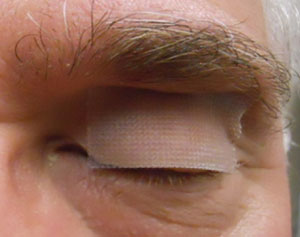
Figure 2. A temporary tape-tarsorrhaphy over the lid crease, narrows the eye opening,
keeps the bandage lens centered and minimises discomfort. Exposure may also cause
undesirable thinning of the amniotic membrane tissue.
Case study
A 56-year old Caucasian female presented at Ophthalmic Consultants of Connecticut for review of a persistent corneal ulcer left eye (OS), managed with a bandage contact lens. On this occasion she reported waking with her ‘lids were glued shut,’ with discharge and swelling around her eyes. She has a previous ocular history of microbial keratitis after sleeping in her coloured contact lenses, and OS recurrent corneal erosion (REE).
Clinical Findings
Unaided visual acuity (VA): right eye (OD) 20/60, pin-hole (PH) 20/40+ and OS 20/200 (with bandage contact lens), PH 20/40. Anterior slitlamp examination revealed OS superior recurrent corneal erosion and inferior corneal neovascularisation. Diagnosis was a REE of the OS. A ProKera Amniotic bandage contact lens was placed on the patient’s eye. Artificial tears as needed, both eyes and Besivance for the left eye every night before dinner was chosen as the most beneficial treatment strategy.
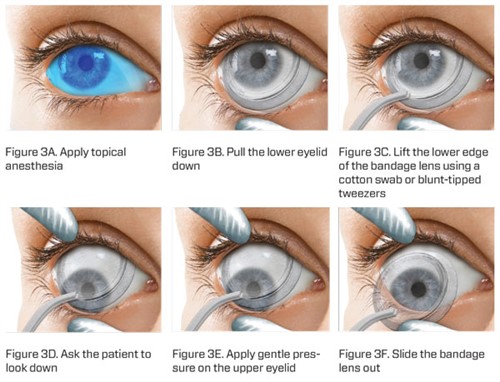
Figure 3A-F. Biological contact lens removal.
Discussion
ProKera is an alternative treatment option to increase the rate and healing of the corneal surface in recurrent corneal erosion. This patient was slow to respond to previous treatment regimens of artificial tears, antibiotics, steroids and rigid contact lenses, therefore this strategy was employed. An amniotic membrane is an avascular, sterile, fetal membrane found deep within the placental tissues during embryological development. It contains an epithelium, stroma and basement membrane. The basement membrane contains collagen, including collagen type VII,2 which is common to the corneal basement membrane and conjunctiva. Amniotic membranes are extracted from donors undergoing caesarean delivery and are analysed for any transmissible diseases and treated with broadspectrum antibiotics prior to ocular application.2
When applied to the cornea, the amniotic membrane – either cryogenic or dehydrated – interacts with the surface to promote epithelial growth through cell migration, differentiation and adhesion to the ocular surface.2,4 It also promotes limbal stem cell regeneration and repair of the corneal epithelium.
The stromal portion of the amniotic membrane contains hyaluronic acid which is effective in inhibiting fibroblast growth, decreasing cytokine expression and therefore decreasing inflammation4 and angiogenesis.2 It is also believed that the amniotic membrane displays antimicrobial properties which is useful in decreasing the chance of corneal infection.
How the amniotic membrane provides anti-microbial defence is still a topic of controversy, but it is suggested that it is due to components within the tissue and the bandage contact lens itself acting a physical barrier between the eye and the environment.2,4
Implantation of the ProKera from a donor to recipient does not mount an immune response as it lacks major histocompatibility antigens HLA-A, B and/or DR.2,4 Despite this, with inefficient sterilising of the amniotic membrane prior, there is still a possibility of transmitting a viral or bacterial infection to the host if the donor amniotic membrane is not accurately screened or stored correctly.4
In 2009, a study investigated the safety and efficacy of ProKera for patients with anterior eye disease. Out of the twenty participants (twenty eyes), 60 per cent recovered visual acuity over an average of twenty-five days. Possible side effects were recurrence of the eye conditions in 20 per cent and 30 per cent reported ocular discomfort and headache. Discomfort is most likely due to the ProKera polycarbonate ring, especially from the superior eyelid.2,5 Researchers suggest that the application of a softer ring may be a way to improve ocular comfort.5
Although currently unavailable in Australia, the use of biologic corneal bandage devices in the US have provided safe and effective healing to corneas with less scarring and inflammation, less pain and improved clinical outcomes.
ProKera offers clinicians a safe and effective alternative approach to manage ocular surface disorders such as RCE. It may be useful for patients with chronic eye diseases where traditional therapeutic approaches are not enough.
*Amniotic membranes currently do not have widespread, private practice, commercial availability in Australia compared with the USA. Access in some states is via the cornea bank or major eye hospitals.
1. ProKera [Internet]. Miami: Bio-Tissue; 2016 [cited on 2017 Nov 30]. Available from: http://www.biotissue.com/patients/our-products/patients-prokera.aspx.
2. Mcgaughy AG, Gupta PK. In-Office Use of Amniotic Membrane. Fekrat S, Scott IU (eds.). American Academy of Ophthalmology. 2015. [cited on 2017 Nov 30]. Available from: https://www.aao.org/eyenet/article/in-office-use-of-amniotic-membrane
3. Department of Health & Human Services. 510(k) SUMMARY ProKeraT M Bio-Tissue, Inc. [Internet]. Washington: U.S. Food and Drug Administration; 2003 Dec 12 [cited 2017 Nov 30]. Available from: https://www.accessdata.fda. gov/cdrh_docs/pdf3/k032104.pdf.
4. Malhotra C, Jain AK. Human Amniotic Membrane Transplantation: Different Modalities of its use in Ophthalmology. World J Transplant 2014: 4; 111-121.
5. Pachigolla G, Prasher P, Di Pascuale MA, et al. Evaluation of the role of ProKera in the management of ocular surface and orbital disorders. Eye Contact Lens 2009; 35: 172-175.
Tagged as: Contact lenses, Pharma